Ferhat Ozturk
As we live our lives, we often come across problems that can block our way. If our car leaks oil, stalls, or breaks down on the road, we immediately bring it to the mechanic to get it fixed. He either replaces the defective part or reinforces it with some additional nuts and bolts. What if our body leaks unwanted fluids into different organs or lacks the required mechanism to produce the essential liquids that our systems need? What if this is a problem that has been inherited from our parents or that will be transferred to our children; what if we are not even aware that we have such a disorder? Or suppose that the normal mechanism in our vital organs is disrupted by foreign invaders, such as cancer cells? We (or scientists) have to find a way to treat these life-threatening problems as soon as possible; otherwise there is no mechanic (doctor) who will be able to fix our organs when they have been severely damaged due to unavoidable defects.
Almost all of us are familiar with the fact that our bodily organs are composed of tissues, which are made of cells. The perfect machinery of the cells is controlled by our genes; i.e. DNA and RNA. Therefore, a minor defect or mutation in the genetic code may affect either partial or entire systems within the body. Some of these genetic diseases are inherited from our parents or relatives. Others may be introduced into our body through environmental mutagens, such as ionizing radiation, ultraviolet rays, or different chemicals within our food and drink; these can result in cancer or cardiovascular diseases. We can protect ourselves from the latter by using appropriate outfits, taking care with our diet, etc. However, inherited genetic disorders are usually unavoidable and sometimes have fatal consequences. Modern medicine is striving to find a way to treat these diseases. Although there are some medical procedures that may lessen the pain of patients or extend their life expectancy, there is no available comprehensive curative therapy for genetic disorders.
Gene therapy has become one of the rising stars in the field of molecular medicine during the last decade, with more than 1500 proposed or ongoing clinical trials worldwide. Gene therapy promises to provide curative therapies for a large number of inherited or acquired diseases, such as monogenic disorders, cancer, or cardiovascular disease. Gene therapy is universally defined as the replacement of an abnormal/dysfunctional gene in the cells of an individual with its correct and functional version. Mechanics use a number of tools to fix our cars for us when they give us trouble; in the same way, gene therapy can be used by doctors to alleviate or completely eradicate some diseases from our bodies.
Gene therapy is classified into two categories based on the target cells that are to be treated. The transduction of the differentiated cells of an individual, i.e. the somatic cells, is known as somatic gene therapy; the transduction of reproductive cells, i.e. gametes (sperm or ova), is known as germ-line gene therapy. Currently, there are many regulations in place that limit the likelihood of the modification of the germline in any gene therapy approach. This is because fear exists that the ability to alter the germline will result in the widespread application of gene therapy to achieve eugenic genetic enhancements, such as improvement of intelligence or physical characteristics. On the other hand, transgenic animals, which are used to detect the function of the genes within an organism, can be generated by modifying the germ-line. Furthermore, gene therapy is also classified into two groups: adult and fetal (in utero) gene therapy, according to the individual to be treated. There are several advantages and disadvantages to these methods, such as immune response, the pooling of mitotic cells, the amount of vector that is required, etc.; however, these are matters for a different article.
Gene therapy is achieved by using special exogenous genetic material transfer agents which are called vectors; these can be compared to the special tools used by auto mechanics. Over the years, a number of gene transfer vehicles, i.e. vectors, have been developed and these can be divided into two principal categories: non-viral (synthetic) and viral (virus-based) gene delivery systems.
Non-viral gene transfer can be achieved by using both physical and chemical methods. The physical methods include: i) Electroporation, in which areas of the cell membrane break down as result of an applied electric pulse, thus allowing DNA to enter the cell, ii) Ballistic gene transfer (the Gene Gun), which bombards particles coated with DNA into the cells, and iii) Microinjection, in which DNA is transferred through microcapillaries into the cells [1]. In terms of chemical gene transfer, lipofection is the most promising method; in this method negatively-charged DNA molecules bind to cationic lipid particles through electrostatic interaction and the DNA–lipid complex enters the cell through endocytosis/pinocytosis. Although these non-viral delivery systems exhibit low toxicity and can be easily produced in high concentrations on a commercial scale, in general, gene transfer using these agents is inefficient and often transient. Therefore, as a result of the viral vectors’ ability to efficiently deliver and integrate genes into the host genome, they are being engineered extensively to achieve a sustained and high-level expression of the gene of interest (transgene).
Viral vectors
Have you ever thought that one of the major pathological agents that cause catastrophic and even fatal diseases could be used to treat the same or a similar disease?
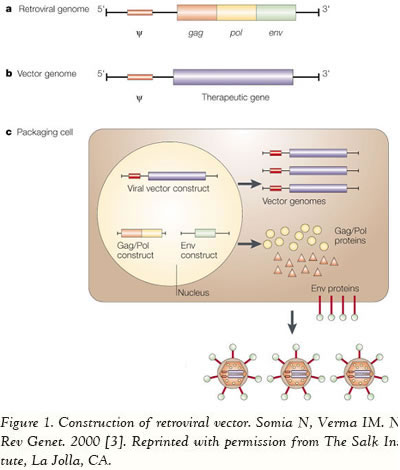
Viruses are equipped with specialized molecular mechanisms that allow them to efficiently transport the genomes into the cells they infect and use the cell’s machinery for their own reproduction. Molecular biologists first harnessed this machinery of transduction in the 1970s. Paul Berg used a modified SV40 virus containing DNA from the bacteriophage lambda to infect monkey kidney cells that were being maintained in culture. Viral delivery systems are based on replicating viruses that have the ability to deliver genetic information into the host cell, a process known as transduction [2]. Due to the fact that there are several advantages to viral vectors, these are the vehicles being employed in approximately 75% of all ongoing clinical trials worldwide. Numerous viruses are being used as the basis for the vectors, including, but not limited to, adenovirus (24%, n=377), retrovirus (20.9%, n=329), adeno-associated virus (4.3%, n=67), herpes simplex virus (3.2%, n=51), vaccinia virus (7.9%, n=124), poxvirus (5.8%, n=91), and baculovirus (more detailed information is available at www.wiley.co.uk/genmed/clinical). The first step to construct a viral vector for transferring the gene of interest involves the identification of the viral sequences that are necessary for replication and pathogenesis. Then some of the genes are removed to make room for the transgene and to render the viral vector replication-incompetent. Consequently, the vector is unable to replicate within the host, and therefore is safe for delivering genes to human cells or tissues (Figure 1).
There are advantages and disadvantages to all of the currently available vectors; the suitability of the vector, therefore, actually depends on the disease or condition that is being treated. For instance, if the goal of the gene therapy is to increase bone marrow engraftment with the transient expression of a growth factor, the use of chemical transfection or naked DNA transfer methods would suffice. However, if the objective is to provide long-term treatment for an inherited disease, then the use of an integrating viral vector is more desirable. Thus, a vector that might be ideal for treating one defect may not be ideal for another. Driven by the desire to develop the “perfect” vector, scientists are continually striving for novel forms of gene delivery that might become the “magic bullet.” Somia and Verma [3] have proposed that the ideal gene therapy vector should include all of the following properties: 1) easy production at a high titer on a commercial scale with a reasonable shelf-life for transport and distribution 2) sustained or regulated expression of the transgene product that is adjustable to the nature of the disease, 3) absence of immune response against the vector and the transgene product, 4) ability to target specific tissues and/or cell types while avoiding professional antigen-presenting cells, 5) absence of size limitations for the genetic material to be delivered by the vector, 6) site-specific integration of the transgene into the chromosome of the target cell to avoid insertional mutagenesis, or faithful division and segregation if it is to reside in the nucleus as an episome independent of local chromatin environments, and 7) an ability to infect dividing and post-mitotic cells. Unfortunately, none of the currently available gene delivery vectors carry all of these features; however, many vectors have enough of the attributes to make them promising for clinical use.
Candidate diseases
During the last 4 decades, gene therapy for many diseases has progressed from preclinical to clinical studies; these range from monogenic recessive disorders such as hemophilia and cystic fibrosis to more complex diseases such as cancer, cardiovascular disorders, human immunodeficiency virus (HIV), neurological and ocular pathologies. The prevalence of diseases that fall under the scope of gene therapy is enormous and most of them have catastrophic or fatal outcomes. Therefore, gene therapy approach for almost any of the diseases mentioned above has an obvious appeal and rationale. To date, more than 1,540 gene therapy clinical trials have been initiated, and these are continuing or have been completed in 28 countries, using more than 100 genes, including antigens, cytokines, tumor suppressors, growth factors, and deficiency genes [4].
Candidate monogenic disorders that are considered to be good candidates for treatment by gene therapy include the hemoglobinopathies, X-linked genetic disorders, amino acid metabolism disorders, and lysosomal and other storage diseases. Currently, there are more than 4000 monogenic diseases registered in the OMIM database. The ultimate aim in treating monogenic diseases with gene therapy is the correction of the disorder by the stable transfer of the functioning gene into dividing cells (stem/progenitor cells), which will ensure the permanence of the correction [5]. The first recognized successful clinical gene therapy trial involved the treatment of 11 children who suffered from SCID-X1 (Severe Combined Immunodeficiency), an X-linked inherited monogenic disorder caused due to a mutation in the common cytokine receptor gamma chain (γc). In these patients, immunity was not fully developed due to the blocking of T-cell and natural killer cell development as a result of mutation. Unfortunately, the trial in SCID-X1 also exemplified one of the potentially serious side effects of gene therapy. Three of the children developed uncontrolled clonal T-cell proliferation, that is, leukemia, almost 3 years after treatment. This case was associated with the integration of the retroviral vector close to the promoter of the LMO-2 proto-oncogene. As a result, LMO-2 protein expression was up-regulated in an abnormal way and resulted in leukemia [7]. Another candidate monogenic disorder for gene therapy is cystic fibrosis (CF), in which abnormally thick mucus is produced in the lungs of the patients, causing difficulty in breathing and increasing the frequency of serious lung infections. CF is known as the most common inherited genetic disease in Europe and USA, especially within the Ashkenazi Jewish population. The average life expectancy of patients with CF is less than 40 years; hence the treatment of this disease has become one of the prime targets of gene therapy research.
In addition to its use in the treatment of monogenic diseases, gene therapy is also becoming a therapeutic alternative for the treatment of various forms of cancer. Indeed, almost 65% of ongoing clinical trials are related to cancer (more detailed information is available at www.wiley.co.uk/genmed/clinical), an area in which much more promise can be seen; this is also a reflection of the urgent need for new therapies to tackle the escalating incidence of this disease. Several different principles are used to treat cancer, including gene therapy that is targeted at tumor suppressor genes, such as p53, or central signaling molecules, as well as "suicide gene" therapy, in which the transgene is capable of converting pro-drugs (selectively less active drugs) into drugs that are toxic for tumor cells. Furthermore, various gene therapy protocols have been developed to strengthen the host’s anti-tumor immune responses by immunotherapy. Most of these studies have been early clinical trials designed primarily as studies of the safety, applicability, and toxicity of gene therapy. Several of these phase I and II studies have, however, shown partial remission of tumors and, in rare cases, complete remission. However, complete cure of the tumor has not yet been achieved. In some trials, including TP53 gene therapy trials, regression in tumor size has been observed in up to 50% of patients. China has become the first country to license gene therapy as a regular treatment for neck and head cancer; here Gendicine, a replication-defective Ad5 vector expressing p53 from a Rous sarcoma virus (RSV) promoter, is used for therapy [6].
In addition to its applications for cancer and monogenic diseases, gene therapy has become one of the favorite methods in cardiovascular research field. This is in step with the rise in clinical trials for cardiovascular gene therapy from 8.3% to 9.1% during the last few years, becoming the second most popular application for gene therapy. In accordance with the variety and occurrence of cardiovascular diseases, different gene therapy strategies have been developed to tackle each disease on its own terms. The expectation is that gene therapy will provide a new avenue for therapeutic applications in the growth of blood vessels, as well as the protection, regeneration, and repair of heart tissue, the prevention of the reoccurrence of constricted or narrowed arteries following cardiovascular intervention, the prevention of the rejection of a bypass, and risk-factor management [4]. Long-term therapeutic gene expression is required in some diseases, such as hypertension research, where reversal and prevention are the key targets. On the other hand, for some other types of cardiovascular diseases, such as ischemia, atherosclerosis, and restenosis, shorter-term gene control will be sufficient to prevent further progress of the symptoms. Therefore, different gene-therapy vectors have to be considered for the treatment of each specific cardiovascular disorder.
Consequently, gene therapy offers new avenues of treatment for diseases including monogenic disorders, cancer, cardiovascular diseases, infectious pathologies and many more. As we follow the tradition, “God did not send down any illness for which He did not also send a cure (Bukhari)”, gene therapy using either viral, non-viral, or any other novel methods may pave the way for the cure of many diseases that are highly prevalent in the world and which for decades have been perceived as untreatable.
Dr. Ferhat Ozturk is a postdoctoral research associate at University of Nebraska Medical Center.
References
- Wells DJ. “Gene therapy progress and prospects: electroporation and other physical methods.” Gene Ther. 2004 Sep;11(18):1363-9.
- Kootstra, N.A. and I.M. Verma, “Gene therapy with viral vectors.” Annu Rev Pharmacol Toxicol, 2003. 43: p. 413-39.
- Somia, N. and I.M. Verma, “Gene therapy: trials and tribulations.” Nat Rev Genet, 2000. 1(2): p. 91-9.
- Edelstein, M.L., M.R. Abedi, and J. Wixon, “Gene therapy clinical trials worldwide to 2007--an update. J Gene Med, 2007. 9(10): p. 833-42.
- http://www.biomedisch.nl/en/gene_therapy_targeted_diseases.php
- Peng, Z., “Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers.” Hum Gene Ther, 2005. 16(9): p. 1016-27.
- Cavazzana-Calvo M, Fischer A, Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest. 2007 June. 117(6):1456-65